Abstract
Background: Hypomethylating agents (HMAs) have changed the treatment landscape for patients with higher-risk myelodysplastic syndromes (HR-MDS) in the past two decades. However, less than half of patients respond to HMA therapy and even the best responses are transient and non-curative. Therapeutic options for those who have failed HMAs are limited, and these patients have very poor prognosis. To speed the development of novel therapeutics, varying response rate definitions and event-free survival (EFS) have been suggested as clinical benefit endpoints for regulatory approvals. However, the relationship between complete remission (CR), partial remission (PR), hematologic improvement (HI), event-free survival (EFS) and overall survival (OS) in MDS has not been conclusively established. To evaluate the relationship between these endpoints, we conducted patient-level response analyses of MDS trials submitted to the FDA.
Methods: We searched for trials submitted with New Drug Applications for treatment of MDS between 2000 and 2020. Trials which supported the efficacy of approved treatments for HR-MDS were included. Patients with chronic myelomonocytic leukemia (CMML), bone marrow blasts ≥ 20%, or missing bone marrow blast information were excluded. A patient-level responder analysis was performed to compare OS and EFS between responders and non-responders, regardless of treatment assignment in the pooled dataset. Separate analyses were formed for CR, PR, and HI. Response rates were defined per protocol; criteria included IWG 2000, IWG 2006, and custom criteria. EFS was defined as time from randomization, or first dose of study drug for single-arm trials, to treatment failure (defined as date of transformation to AML, given availability across trials) or death from any cause. For each trial, the hazard ratio of response vs. no-response on OS and EFS was estimated using a Cox model with response as a time-varying covariate and treatment, sex, age, and percentage blast count as baseline covariates. The resulting hazard ratios were combined assuming a common effect of response on clinical outcome to estimate a pooled hazard ratio. Unadjusted distributions of OS and EFS by response were obtained using the Kaplan-Meier method.
Results: We identified 8 trials with a total of 772 patients with HR-MDS investigating 3 experimental agents (azacitidine, decitabine, and decitabine + cedazuridine) for the treatment of HR-MDS. Three trials were randomized trials to assess efficacy (N=116, 124, 215), two were randomized crossover trials to assess bioequivalence (N=116, 61), and 3 were single arm trials (N=29, 34, 77). Across all trials, the population characteristics were: median age 69 years (range: 23-92); 70% male, 67% White, 29% Asian, 2% Black; 17% High Risk per IPSS, 25% Intermediate-1, 33% Intermediate-2, 2% Low Risk, and 24% unknown. IPSS information was missing for 3 trials (187 patients).
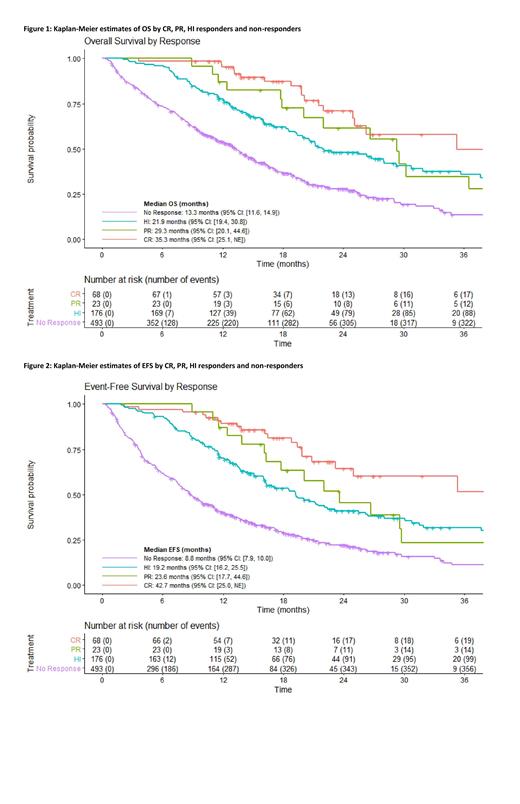
We assessed the effect of CR vs. PR vs. HI in a pooled analysis. Patients who achieved a CR had better OS (HR=0.40 [95% CI: 0.25-0.64]) and EFS (HR=0.39 [95% CI: 0.24-0.63]) compared with non-responders, defined as patients who failed to achieve CR, PR, or HI. Patients who achieved a PR had better OS (HR=0.51 [95% CI: 0.28-0.92]) and EFS (HR=0.66 [95% CI: 0.37-1.17]) compared to non-responders. Patients who achieved an HI response had better OS (HR=0.60 [95% CI: 0.46-0.76]) and EFS (HR=0.60 [95% CI: 0.47-0.76]) compared to non-responders. Patients who achieved a CR+PR had a trend for longer OS (HR=0.73 [95% CI: 0.49-1.11]) and EFS (HR=0.76 [95% CI: 0.51-1.14]) compared with those who achieved HI alone. Between-study variances of these effects were not able to be reliably estimated due to the low number of responses per trial and low number of trials included in the analysis. Figures 1 and 2 present unadjusted Kaplan-Meier estimates of OS and EFS by response group.
Conclusions: Patient-level responder analyses suggest that CR, PR, and HI responders have better OS and EFS compared with non-responders. A limitation of these analyses is that response criteria are not standardized across trials. Future work includes standardizing response criteria across trials. However, our results suggest that CR, PR, and HI may be associated with long-term benefit in patients with HR-MDS. While HI responses had better OS compared to no response, CR and CR+PR remain the response endpoints associated with greatest long-term benefit.
Vallejo: AstraZeneca: Other: Spouse is employed by AstraZeneca..

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal